Applications of laminates made of mono-materials for enteral nutrition and pharmaceutical packaging
How can packaging for pharmaceutical products and enteral nutrition be made more environmentally friendly without compromising on quality and safety?
The targeted use of recyclable materials such as polypropylene (PP) reduces the material mix in packaging and thus improves its recyclability in …
The mono pouch CLEAR: Sustainability through innovation
Sustainability is a central component of everything we do. With innovative mono-material solutions, we are revolutionizing the packaging industry by developing …
FRÜH @ PharmaPack, Arab Health and MD&M West
Visit us in Paris, Dubai or Anaheim and find out more about our innovative packaging and contract packaging solutions. Our experts are at your disposal for …
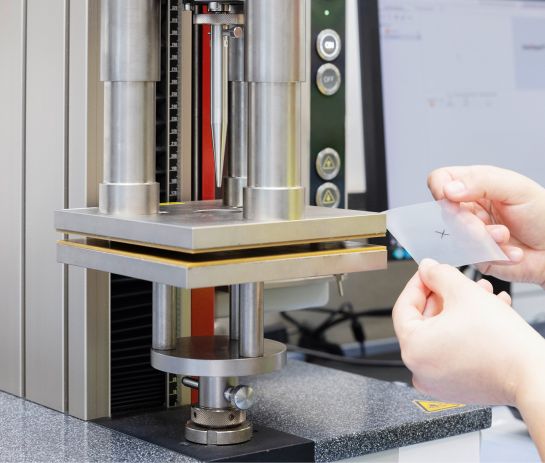
Innovative measuring technology: The new digital measuring projector
In order to continue to guarantee the highest quality and precision, Früh Verpackungstechnik relies on the digital measuring projector from Keyence. This …
Investment in our future: The new BA10 production machine
The BA10 production machine, in use at Früh Verpackungstechnik AG since December 2023, expands our capacities in the production of high-quality pouch systems. …
Visit us at CPhI Milano
We look forward to welcoming new and familiar faces at booth 20D85. Do you have a new project or simply need some initial advice? Our experts will be happy to …
Re-certification: ISO 13485 / ISO 9001 / ISO 22000
We are happy to inform you that we successfully passed the re-certification of our site in Fehraltorf, Switzerland for the following Quality Management System …
MD&M South and Medtec 2024
Visit us in Stuttgart and/or Charlotte in June and discuss packaging solutions in medical technology with our specialists on site.
MD&M South, Charlotte (NC), …
Our Services
Our clients range from large international corporations to recently founded start-ups, whose expectations and needs are often very different. Our lean …
Recyclable material with high barrier for pasteurization and sterilization applications
Discovering the power of mono-packaging
Mono-packaging material, a game-changer in terms of sustainability, refers to the use of a single type of material for …
Mastering Process and Packaging Validation – ISO 11607
- ISO 11607 – 2 covers the requirements for the processes of forming, assembly and sealing of the packaging. The processes involved are known as a (packaging) proc …
Gold status for stand construction at Pharmapack
We were awarded Gold status for our sustainable stand construction at Pharmapack. Thanks to the multiple use of the graphic wall, the screen, the rental …
Quality standards
We subject our qualified facilities and environmental conditions as well as all production processes to a continuous validity check with the following GMP …
Validation
Hygiene validation
Thanks to our many years of expertise in packaging pharmaceutical and medical devices under controlled clean room conditions according to ISO …
- Category:
- Exhibitions/Events White Papers FRÜH-Know-how
- Year:
- 2025 2024 2023 2022 2021 2020 2019